PRODUCTS OF VAPOR OXIDATIVE AMMONOLYSIS OF 4-CHLORO-o- XYLENE AND THEIR CHROMATOGRAPHIC ANALYSIS
Authors
Guliyev Fikret Ali oglu, Bagirzade Gulu Ahmed oglu
Share
Authors
Guliyev Fikret Ali oglu, Bagirzade Gulu Ahmed oglu
Share
Functionally substituted aromatic nitriles, including 4-chlorophthalonitrile, are perspective raw materials for the production of dyes, heat-resistant polyimide polymers and other important products [1]. Gas-phase catalytic oxidative ammonolysis is a single-stage efficient method for the synthesis of imides and aromatic nitriles [2]. Previously, the vapor-phase oxidative ammonolysis of o-xylene on a V-Sb-Bi-Cr/γ-Al2O3-oxide catalyst was studied to obtain phthalimide [3], as well as 4-bromo-o-xylene [4] and 4-phenyl-o-xylene [5] on the V-Sb-Bi-Zr/γ-Al2O3-oxide contact to obtain the corresponding phthalonitrile. It is important to add that, based on kinetic data, a mechanism for the formation of products of oxidative ammonolysis of both 4-bromo-o-xylene [6] and 4-phenyl-o-xylene [7] was proposed at this contact. We [8] indicated the impossibility of comparing the transformations of 4-phenyl-, 4-bromo- and o-xylene during oxidative ammonolysis within the same reaction series. Therefore, a comparison under the same conditions of the reactivity of 4-phenyl-, 4-bromo- and o-xylene in vapor-phase oxidative ammonolysis was studied. To assess the reactivity, the conversion values of the starting materials were used and it was determined that these aromatic compounds are located in the following order in terms of reactivity: o-xylene> 4-phenyl-o-xylene> 4-bromo-o-xylene. An explanation is given that due to the prevailing role of the steric factor of the bulky phenyl radical in the adsorption stage and the electronic factor of the bromine atom in the kinetic region, it is impossible to compare the transformations of 4-phenyl-o-xylene and 4-bromo-o-xylene within the same reaction series, which determined the heterogeneity of the limiting stage and polar effects in these complex processes. For both substituted o-xylene in the process of oxidative ammonolysis, the kinetic control is not of the same type and, therefore, the applicability of the free energy linearity principle (FEL), i.e. the eligibility of using ρ-σ analysis or the proportionality (additivity) of the action of the electronic effects of substituents within the same reaction series is not fulfilled. However, when comparing under the same conditions the reactivity of o-xylene, 4-chloro- and 4-bromo-o-xylene in heterogeneous catalytic oxidative ammonolysis on a V-Sb-Bi-Zr/γ-Al2O3-oxide catalyst, the kinetic control is of the same type and, therefore, the actions of the electronic effects of substituents within the framework of one reaction series are performed [9]. In [10], the reactions of the vapor-phase oxidative ammonolysis of 4-chloro-o-xylene on oxide catalysts were studied and it was shown that, on all samples of catalysts, the chromatographic method identified as reaction products 4-chlorobenzonitrile, 4-chloro-o-tolunitrile, 4-chlorophthalonitrile, 4-chlorophthalimide and carbon dioxide. Compared with other oxides of metals of variable valence, upon modification of the base V-Sb-Bi/γ-Al2O3 oxide catalyst ZrO2, the parameters of the process of oxidative ammonolysis of 4-chloro-o-xylene to obtain 4-chlorophthalonitrile turned out to be higher. Indeed, in the oxidative ammonolysis of 4-chloro-o-xylene on a V-Sb-Bi-Zr/γ-Al2O3 oxide catalyst, 4-chlorophthalonitrile is obtained with a selectivity of 86.42% at a 97.6% conversion of the starting 4-chloro-o-xylene (co-xy), i.e. the yield of the target dinitrile is 84.35% at T= 673K, contact time 1.87 s, Pco-xyo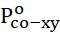 = 1.24 kPa, PО2o
= 1.24 kPa, PО2o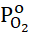 = 7.80 kPa and PNH3o
= 7.80 kPa and PNH3o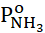 = 61.91 kPa. In this case, 4-chlorophthalimide is formed with a yield of 2.88%, which is considered the second main product along with 4-chlorophthalonitrile. Since ortho-substituents create a steric tension that favors intramolecular cyclization, due to which an imide is formed from the substrate and dinitrile and under the influence of the polar effect, isolated transformations of xylenes occur with the formation of dinitriles [11]. The yields of the main products, being highly dependent on the process conditions and the catalyst used, are determined by the structure of the initial and intermediate substances [12]. Kinetic measurements were carried out on a setup with a 20 cm3 non-gradient flow reactor made of 12Kh18N10T steel with a vibrofluidized catalyst bed. To prevent condensation of high-boiling products, a part of the installation was thermostated at 520–540 K. Oxygen and nitrogen were purified from traces of organic compounds and dried before use. Ammonia was passed through an oil filter. We used 4-chloro-o-xylene of chemically pure grade. 4-chlorophthalonitrile, 4-chlorophthalimide, 4-chloro-o-tolunitrile, and 4-chlorobenzonitrile were isolated from the products of oxidative ammonolysis of 4-chloro-o-xylene and purified by distillation [13].
= 61.91 kPa. In this case, 4-chlorophthalimide is formed with a yield of 2.88%, which is considered the second main product along with 4-chlorophthalonitrile. Since ortho-substituents create a steric tension that favors intramolecular cyclization, due to which an imide is formed from the substrate and dinitrile and under the influence of the polar effect, isolated transformations of xylenes occur with the formation of dinitriles [11]. The yields of the main products, being highly dependent on the process conditions and the catalyst used, are determined by the structure of the initial and intermediate substances [12]. Kinetic measurements were carried out on a setup with a 20 cm3 non-gradient flow reactor made of 12Kh18N10T steel with a vibrofluidized catalyst bed. To prevent condensation of high-boiling products, a part of the installation was thermostated at 520–540 K. Oxygen and nitrogen were purified from traces of organic compounds and dried before use. Ammonia was passed through an oil filter. We used 4-chloro-o-xylene of chemically pure grade. 4-chlorophthalonitrile, 4-chlorophthalimide, 4-chloro-o-tolunitrile, and 4-chlorobenzonitrile were isolated from the products of oxidative ammonolysis of 4-chloro-o-xylene and purified by distillation [13].
In the reaction products, 4-chlorophthalonitrile, 4-chloro-o-tolunitrile, 4-chlorophthalimide, 4-chlorobenzonitrile, CO2 and unreacted 4-chloro-o-xylene, oxygen, and diluent gas nitrogen were identified by chromatography. Chromatographic analysis was carried out according to the following scheme. The reaction gases were passed successively through 1, 4-dioxane traps to absorb nitriles, 4-chlorophthalimide, and 4-chloro-o-xylene and sulfuric acid to absorb ammonia. Analysis of carbon dioxide was carried out on an LKhM-8MD chromatograph. TEGNM on INZ-600 was used as a stationary phase. The separation of O2 and N2 was carried out on the same chromatograph on a parallel column with NaX. The ammonia concentration at the outlet of the reactor was determined from the results of titration of unreacted sulfuric acid in the second trap. Analysis of the products absorbed by 1, 4-dioxane was carried out on a Khrom-5 chromatograph with a flame ionization detector. As a stationary phase, which was filled in a column 1200 mm long and 4 mm in diameter, a mixture of Apiezon L (21%) and PEG-40000 (0.5%) was used on N–AW chromaton (0.2–0.25 mm) or polysorb-1 alone (0.25–0.5 mm). The flow rate of the carrier gas (nitrogen) is 80 ml/min. Sample inlet temperature 353 K, programmed temperature rise rate 20 deg/min. Chromatograms were calculated using the internal standard method (label-tetradecane).
References:
1. Ризаев Р.Г., Шейнин В.Е., Магеррамова З.Ю., Багирзаде Г.А., Гусейнов И.А., Гейдарлы Н.И. Тез. докл. научной конференции, посвященной 90-летнему юбилею чл.- корр. НАНА З.Г.Зульфугарова. – Баку. 2004. С. 39.
2. Bagirzade G.A., Guliyev F.A., Tagiyev D.B. European Journal of Analytical and Applied Chemistry. – 2017. № 1. P. 39-43.
3. Bagirzade G.A., Tagiyev D.B., Manafov M.R. International Journal of Current Research. – 2015. Vol. 7. Issue 10. P. 21637-21640.
4. Багирзаде Г.А. Журнал общей химии. 2010. Т. 80. Вып. 8. С. 1360-1364.
5. Bagirzade G.A., Tagiyev D.B., Manafov M.R. Modern Research in Catalysis. –2015. Vol. 4. No. 3. P. 59-67.
6. Багирзаде Г.А. Журнал общей химии. 2014. Т. 84. Вып. 6. С. 896-901.
7. Bagirzade G.A., Tagiyev D.B., Manafov M.R. Advances in Chemical Engineering and Science. – 2015. Vol. 5. No. 4. P. 430-440.
8. Bagirzade G.A. European Journal of Technical and Natural Sciences. – 2019. No1. P. 36-40.
9. Багирзаде Г.А., Кулиев Ф.А. Proceedings of the IX International Scientific and Practical Conference “World science: problems, prospects and innovations”. - Toronto. – 2021. P. 161-170.
10. Багирзаде Г.А., Кулиев Ф.А. Сборник тез докл. V Международной конференции «Техническая химия. От теории к практике». – Пермь. 2016. С. 66.
11. Багирзаде Г.А., Кулиев Ф.А., Тагиев Д.Б. Сборник тез докл. V Международной конференции «Техническая химия. От теории к практике». –Пермь. 2016. С. 67.
12. Багирзаде Г.А., Кулиев Ф.А. Сб. тезисов VII Всероссийской конференции с международным участием «Техническая химия. От теории к практике», посвященной 50-летию академической науки на Урале. – Пермь. 2022. С. 140.
13. Багирзаде Г.А., Кулиев Ф.А. Сб. тезисов VII Всероссийской конференции с международным участием «Техническая химия. От теории к практике», посвященной 50-летию академической науки на Урале. – Пермь, 2022. С. 141.


